Nanoparticles for nucleic acid delivery
Nanoparticles are versatile tools for the delivery of small molecules, like liposomes carrying encapsulated anticancer drugs, but also to deliver nacromolecules like nucleic acids. In the latter case, the extended structure of the nucleic acid can be condensed into nanosized structures and at the same time protected from nucleases, enzymes which can rapidly degrade free nucleic acids. Nucleic acids, like DNA, messenger RNA (mRNA) and small interfering RNA (siRNA) bear a negatively charged phosphate backbone, which, by virtue of electrostatic interaction, can bind to and condense with (poly)cationic molecules. Within the body cell, DNA is condensed by histones, i.e. positively charged proteins. Initial studies with purified or recombinant histones were already conducted in the 1960ies. Also, polyaminoacids like poly-lysine (bearing a net positive charge in the side chain) are used for condensation. Later on, a multitude of cationic polymers (polycations), dendritic structure (e.g. amine-terminated polyamidoamine dendrimers) or cationic lipids (lipids with a mono- or oligocationic head group) have been developed for such purposes (Eliyahu 2005) Per definition, particles consisting of nucleic acids and polycations are termed polyplexes, whereas condensation of nucleic acids with cationic lipids results in lipoplexes.
The process of nanoparticle formation is influenced by several factors, like the ratio of positive charge in the polycation and the negative charge in the nucleic acid, the ion concentration (presence of salt), the pH and the overall concentration of polymers present in solution. Hence, this process had to be properly controlled to obtain a homogeneous, stable nanoparticle preparation. Mixing of components can be achieved simply by pipetting or, for up-scaling the production, in a controlled way using micromixer devices (Taschauer 2016). In any case, biophysical parameters like particle size, colloidal stability and surface charge have to be monitored. We apply transmission electron microscopy (TEM), LASER light scattering, surface plasmon resonance (SPR) measurements, nanoparticle tracking analysis (NTA) and zeta potential measurements to monitor these parameters (Taschauer 2020).
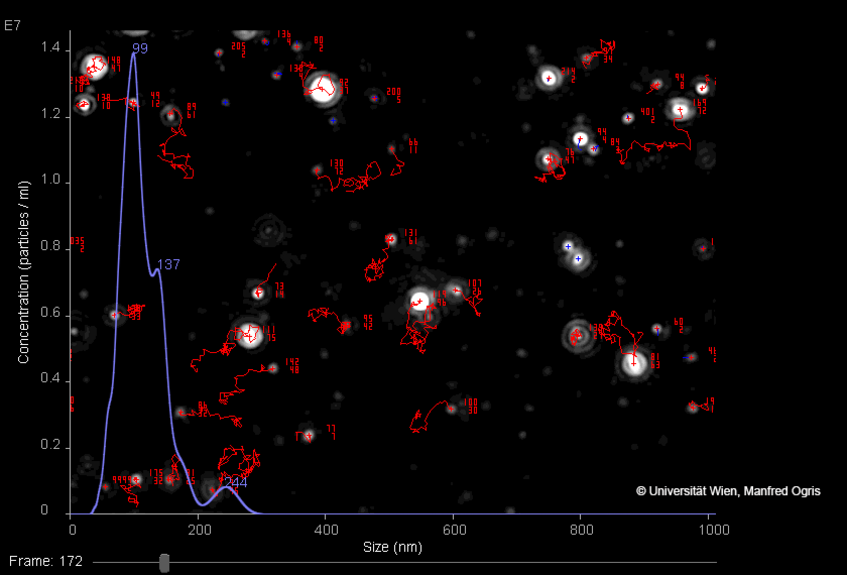
The Brownian motion of individual nanoparticles is tracked by detecting the laser light scattered by each particle (white dots: nanoparticles, red lines: trajectories of individual particles). Several frames are recorded with a video camera. Size data of a whole particle population is depicted as histogram (lilac line). Similarly, when applying an electric field, the zeta potential (surface charge) of nanoparticles can be detected. As NTA measures individual particles, a high quality and high resolution measurement is possible also in samples with high polydispersity (i.e. broad size distribution, presence of aggregates).
Polycations, polyplexes and endosomal release
Polyplexes and most other nanoparticles for nuclei acid delivery are generated with a surplus of positive charge to ensure their colloidal stability. Such positively charged particles usually interact with cells by interaction via negatively charged cell surface components, like glycoproteins (e.g. heparan sulfate proteoglycans). After binding, they are internalized by adsorptive endocytosis, were the actin cytoskeleton is dragging the endosomal vesicle containing nanoparticles into the cell. Here, a major hurdle exists for successful transfection, namely the release of the payload from the endosome. Viruses have developed several mechanisms to enable access of their nucleic acid to the cytoplasm. Certain enveloped viruses, like HIV1, directly fuse with the plasmamembrane, a process which is triggered by distinct viral proteins (Chen 2020). SARS-CoV2, another enveloped virus, can also directly fuse with the plasmamembrane of the cell or with the endosomal membrane, once the virus in internalized (Peng 2021), while influenza virus carries a protein (Hemagglutinin), which destabilizes the membrane once the endosome is acidified by the ATP driven proton pumps (Lim 2020). Polyplexes face a similar hurdle to access the cytoplasm after internalization into endosomes or other intracellular vesicles. Here viral mechanisms can be harnessed, especially when considering the decrease in pH in the endosome after particle internalization. For example, synthetic peptides derived from influenza virus hemagglutinin can be attached to a polyplex and promote endosomal escape (Zhang 2016). Several polycations bear an intrinsic endosomolytic, i.e. endosomal release activity, like polyethylenimine (PEI). One hypothesis to explain this effect is the so called proton sponge effect (see figure below).
PEI carries either a mixture of primary, secondary and tertiary amines (when in the branched form, BPEI) or secondary amines only (when in the linear form, LPEI). When polyplexes are formed at neutral pH (7.4), only a fraction of amines is protonated, i.e. positively charged. Once the polyplex enters the endosome and the pH decreases in the endosome due to ATP-driven proton pumps, the incoming protons (H+) are utilized to protonate the residual, not yet protonated amines leading to a buffering of the pH. Subsequently, a flow of Chloride ions (Cl-) from the cytoplasm into the endosome increases the ion concentration. This then leads to an osmotic effect with water influx indo the endosome, the vesicle expands and subsequently bursts releasing its payload, including the nucleic acid delivered, into the cytoplasm. Already within the endosome, the polyplex can be destabilized, and after release into the endosome the nucleic acid is released from the polymer (as it has been observed for LPEI, (De Bruin 2008). However, also other mechanisms have been discussed which lead to endosomal release, like pore formation or membrane destabilization by direct interaction with the polycation (Pei and Buyanona 2019)
In our projects, we mostly use LPEI and low molecular weight derivates thereof for the generation of polyplexes and apply them for local and targeted nucleic acid delivery.
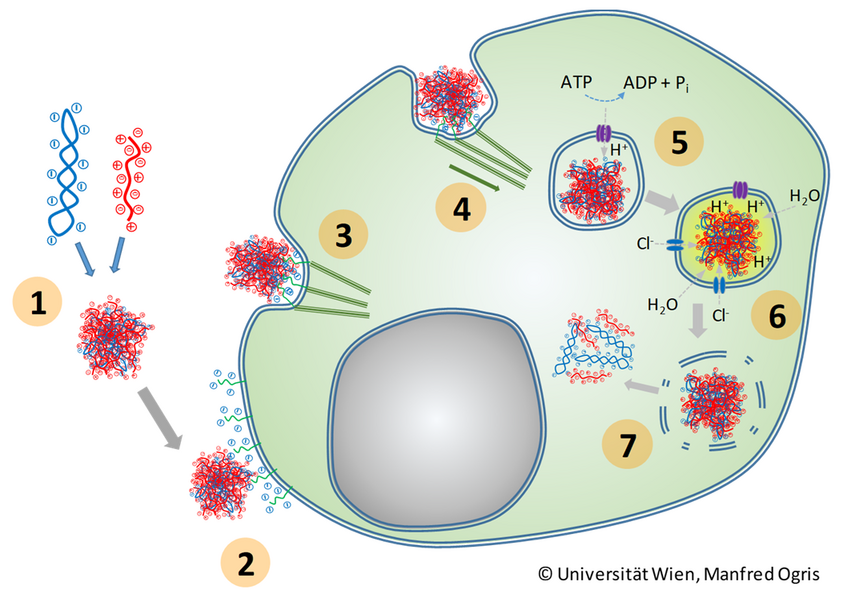
Polycations (e.g. LPEI) and nucleic acid (e.g. plasmid DNA) form polyplexes by virtue of electrostatic charge interactions (1); polyplexes interact with negatively charged cell surface glycoproteins (heparane sulfate proteoglycans, syndecans) (2); actin fibers attach to the intracellular domain of membrane proteins (3) and drag polyplexes into the cell by forming endosomal vesicles (4); ATP driven proton pumps start to acidify the endosome (5); polycations absorb the protons acting as a ‘protong sponge’; this leads to swelling of the polymer, destabilization of the polyplex and influx of chloride as counter ions (6); finally the osmotic imbalance leads to influx of water, subsequent bust of the endosome and release of its payload into the cytoplasm (6)
