Peptide ligands for receptor targeting of polyplexes
Nanoparticle for nucleic acid delivery based on polycations or cationic lipids usually bear a net positive surface charge. This permits binding to the negatively charges cell surface and triggers uptake by adsorptive endocytosis. While such positively charged nanoparticles are suitable for application in vitro, ex vivo or for certain localized applications in vivo, systemic application of polyplexes often triggers their aggregation with blood components, like blood platelets, erythrocytes and plasma proteins. Particles are then entrapped in the first vascular bed encountered, namely the lung (Ogris 1999). Stabilizing nanoparticles, preventing their interaction with blood components and increasing their circulation halt life in blood have been central aspects in the development of tumor targeted therapies. The hydrophilic polymer poly(ethylene glycol) (PEG) is successfully used to improve drug delivery into tumors. PEG-liposomes loaded with anticancer drugs, e.g. doxorubicine, show an extended circulation in the blood stream, reduced heart toxicity and improved tumor accumulation. This can be explained by the so called enhance permeability and retention (EPR) effect, albeit also other effects are involved (Shi 2020). Here, PEG prolongs the circulation time of the drug carrier in the bloodstream by preventing protein binding and cellular clearance. The leaky vasculature together with an incomplete lymphatic drainage promotes the accumulation of liposomes in the tumor. In a similar way, PEG can be used for nucleic acid based nanoparticles, were it also shield the net positive surface charge of the polyplexes. While this PEG-shield leads to a de-targeting of polyplexes, subsequent re-targeting to specific surface receptors can be achieved when incorporating targeting ligands into polyplexes (Schäfer 2011). Protein ligands, like growth factors, transport proteins or antibodies allow a high affinity binding to cellular receptors often overexpressed on cancer cells. However, their extended molecular weight can negatively interfere with the complexation process. Also, growth factors like epidermal growth factor (EGR) can act as a mitogen, i.e. promoting cell growth and division. This would be counteractive for an anticancer approach.
We use small, fully synthetic peptide ligands for targeting purposes. Peptides are synthesized containing the amino acid cysteine, which bears a free thiol group. Coupling to LPEI is achieved with a heterobifunctional PEG linker and the conjugate purified by ion exchange chromatography (Rödl 2019). Such conjugates have already been successfully applied in vivo, e.g. for targeting the cell surface protein CD49f, which is overexpressed in many solid cancers (Taschauer 2919).
In ongoing projects, we develop novel peptide ligands with improved receptor affinity applying in silico modeling, chemical conjugation and affinity studies directly on immobilized receptors and on cultures cells in vitro.
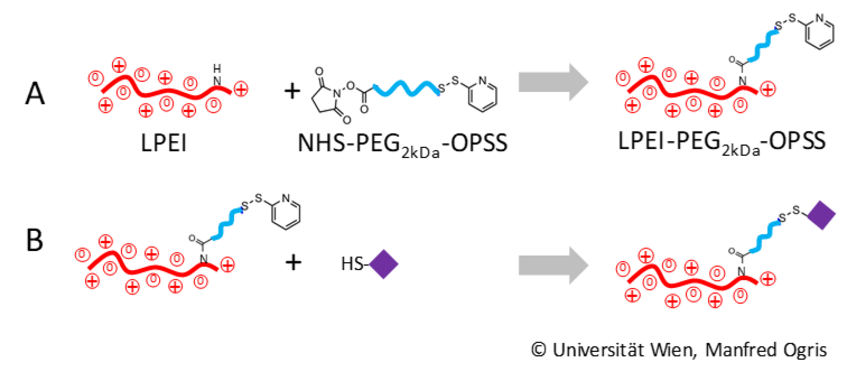
LPEI is reacted with heterobifunctional PEG carrying an amine-reactive N-hydroxysuccinimid group (NHS) and a thiol-reactive (3-(2-pyridyldithio) propionamide) (OPSS). After formation of a stable amine bond with an amine group in LPEI, the resulting conjugate LPEI-PEG-OPSS is purified and quantified by 1H-NMR (A). In a second step, LPEI-PEG-OPSS is reacted with peptide containing a cysteine with a free thiol group. The peptide is bound via the distal OPSS group forming a disulfide-bridge. The resulting LPEI-PEG-peptide conjugate purified and characterized (B). © Universität Wien, Manfred Ogris
